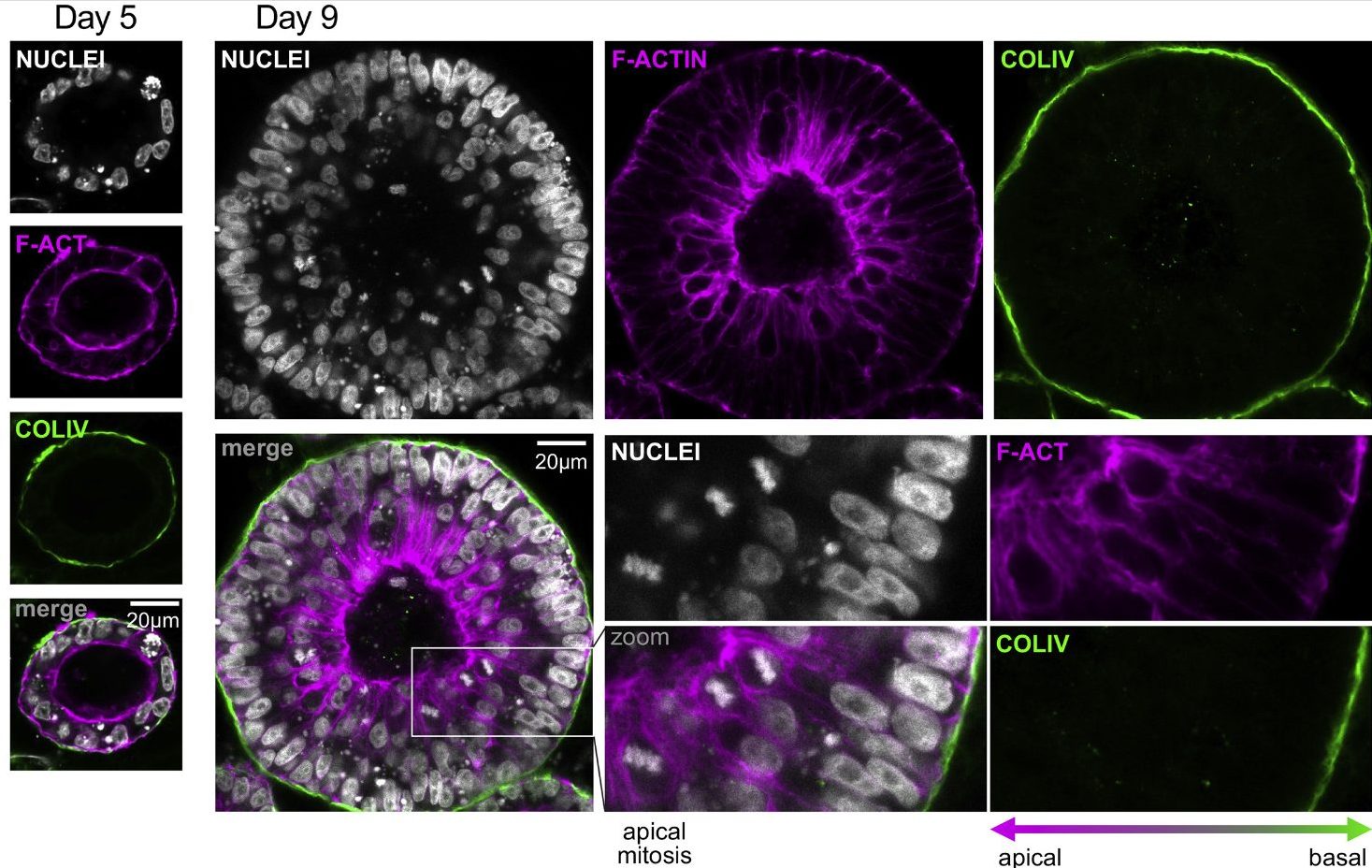
Our latest experimental evidence pointed out the importance of controlling the starting point (i.e. the human state of pluripotency) to achieve a proper human morphogenesis in vitro. Thus, we combine reprogramming of somatic cells with morphogenesis of induced pluripotent stem cells to create engineered human-specific in vitro models of early stages of embryonic development.
The possibility of reprogramming human adult somatic cells to an embryonic-like state, called induced pluripotent stem cells (iPS) offers breakthrough perspectives for research and clinical applications, including modelling of inaccessible stages of human embryonic development, modelling of genetic and epigenetic diseases, drug efficacy and safety screening on patient derived target tissues.
In this scenario, we developed robust and efficient techniques based on microfluidics to reprogram adult somatic cells back to two distinct pluripotent stages, named naive and primed, which in vivo correspond to the pre-implantation and the post-implantation pluripotent epiblast cells, respectively (Gagliano et al., Nat Prot. 2019; Giulitti et al., Nat Cell Biol 2019; Panariello et al., Nat Comm 2023).
The efficient conversion of somatic cells towards the ground state of pluripotency (i.e. naive) offers an unprecedented possibility of modelling some genetic and epigenetic conditions that can’t be modelled using conventional primed iPSCs.
However, the usage of naïve cells is still restrained by two main factors: the usage of a feeder-dependent culture method and the very inefficient differentiation.
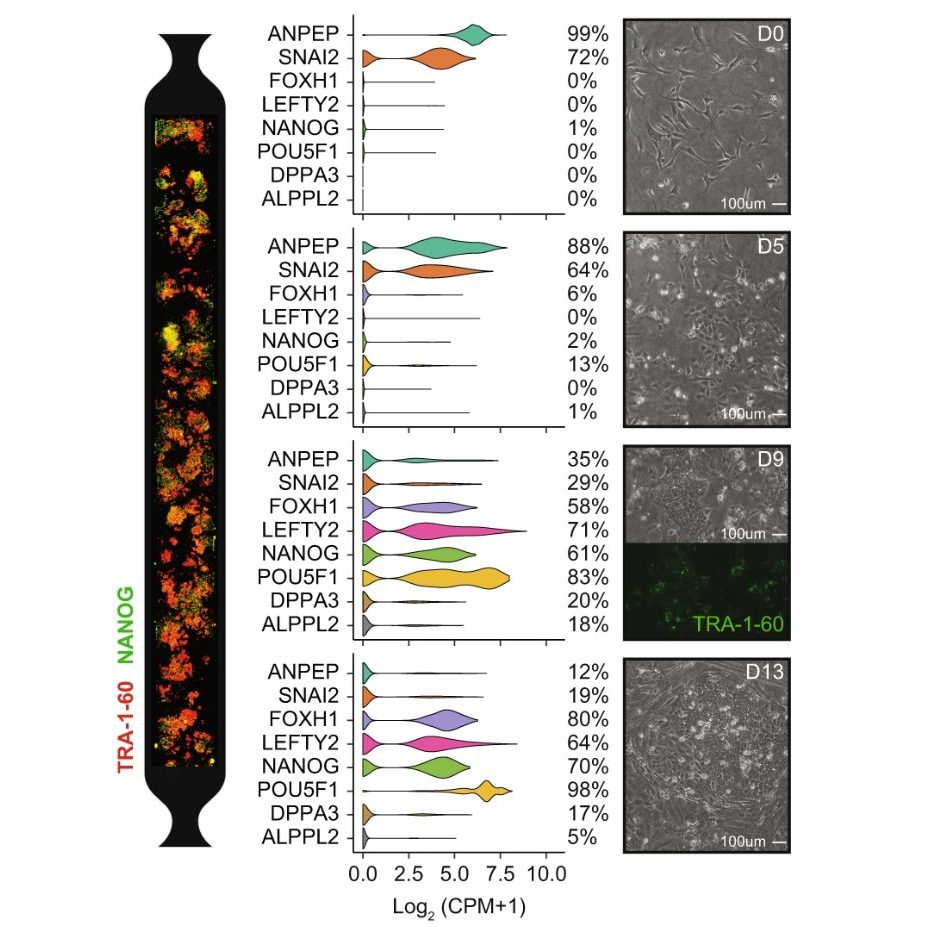
Dynamics of identity acquisition during reprogramming in microfluidics (Panariello et al., Nat Comm 2023)
We set up an innovative expansion method for naïve cells in feeder free conditions and in 3D (Cesare et al., Cell Stem Cell), which is functional for any further application of human naïve cells.
By using an unbiased and integrated approach we found that naïve, but not primed, hiPSC colonies are characterised by an extracellular matrix-rich microenvironment, and by a specific extracellular matrix fingerprint at transcriptional, proteomic and secretome levels. Based on this, we developed a 3D culture system, based on extracellular matrix (ECM)-rich hydrogel, that supports robust longterm feeder-free self-renewal of naive hiPSCs and also allows direct and timely developmental morphogenesis simply by modulating the signalling environment.